Water Electrolysis Producing Green Hydrogen
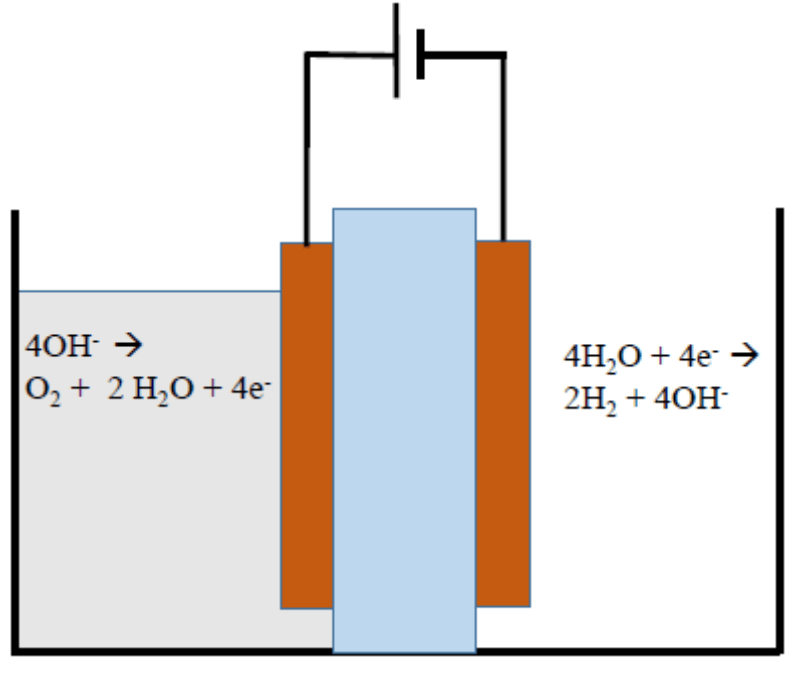
Renewable energy can be used to supply significant global energy and energy independance. However, renewable energy can be intermittent and requires an economical way of storing and converting the energy to a chemical form. Hydrogen produced via water electrolysis is a means of on-site chemical energy storage for renewable energy sources. Green hydrogen can be transported and used in chemical synthesis, heat engines or fuel cells. Alkaline low-temperature electrolysis (LTE) systems enjoy several potential advantages over acid-based LTE systems including facile oxygen evolution reaction (OER) kinetics and electrodes that use no (or little) platinum group metals (PGM). The polymer membranes and membrane electrode assembly (MEA) structures going into alkaline LTE systems have also seen significant advances in recent years. In this project, we have developed the highest conductivity, chemically-stable hydroxide conducting membrane to-date. The poly(norbornene)-based membrane and ionomers (used to make the electrodes) have extremely high hydroxide density and can be cross-linked to control water uptake. Self-adherent ionomers have been synthesized and shown to give excellent adhesion of the electroactive catalyst to the metal current collector.
Self-Immolative, Transient Polymers
We have developed a unique family of photo-thermal polymers which quickly transform from the solid to the vapor or liquid state. They are used as photo or thermal resists in direct write patterning tools. The polymer resists are composed of cyclic, low ceiling temperature (Tc) poly(aldehydes). Tc is the thermodynamic temperature which separates polymer from monomer. The Tc of our polymers is below room temperature making the polymer meta-stable at room temperature. The resists very rapidly and cleanly decompose into monomer only when triggered during patterning because the mechanism of depolymerization is suppressed. The polymers have a cyclic architecture. Breaking a single bond at room temperature creates two ends, which enable near instantaneous depolymerization back to monomer.
